What happens when ionic compounds dissolve in water. During the process of dissolution the interaction between solvent - solute overcome the interaction between solute - solute.

Why Are Ionic Compounds Soluble In Water Youtube
A brief explanation of what happens when ionic compounds and some hydrogen compounds dissolve in water.
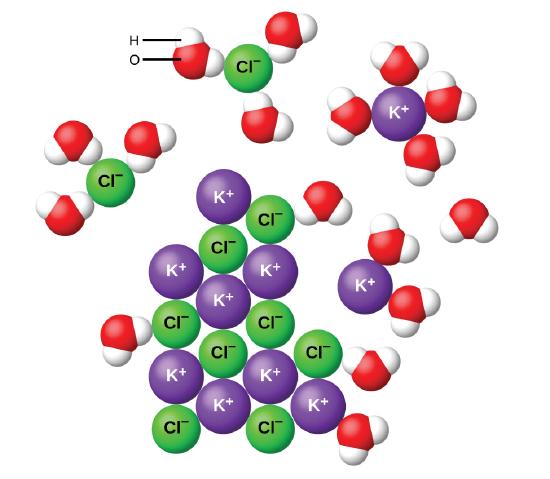
. When some substances are dissolved in water they undergo either a physical or a chemical change that yields ions in solution. Ionic compounds dissolve in water. This reaction is called a double-replacement reaction.
If the attraction between the ions and the water molecules is great enough to break the bonds holding the ions together the compound dissolves. For example when NH4 NO3 is dissolved in water it breaks up into separate ions. When an ionic compound is dissolved in water it breaks into its contituent radical ions.
The water molecules draw positive and negative ions from the crystal as you place an ionic compound in water. 5 What two compounds are electrolytes. If its an Ionic Compound Eg.
So when ionic compounds dissolve in water they separate into cations and anions electrolytic dissociation occurs. A Cations and anions are released into the solution b They stick to the wall of the beaker c They are transformed into non-metals d They split up H2O molecules into Hand O. For example if you take HClwhich consists of covalent bond Though substantial when dissolved in water produces H and Cl- ions which portray a property of ionic compound.
What happens when ionic compounds dissolve in water. If the physical or chemical process that generates the. Through hydrating the ions they do this.
An ionic compound consists of two oppositely charged ions. When this happens the ions dissociate and disperse in solution each. It has a permanent dipole.
Describes what happens when a molecular compound dissolves in water. Describes what happens when an ionic compound dissolves in water. Water is a polar molecule.
When you immerse an ionic compound in water the ions are attracted to the water molecules each of which carries a polar charge. When an ionic compound dissolves in water the positive ends of the water molecules are attracted to the anions and the negative ends are attracted to the cations. 7 Which of the following compounds when dissolved in water.
When ionic compounds dissolve in water the ions in the crystal separate and move throughout the solution. 4 What is a compound that dissolves in water. Differences in electronegativity account for the partial positive charge carried by waters hydrogen atoms and the partial negative charge of its oxygen atoms.
Ionic compounds dissolve in water because the hydrogen and oxygen atoms in the H2O molecules have partial charges that attract the ions in the solid compound causing it to dissociate into separated ions. So when an ionic substance salt dissolves in water it is broken up into individual cations and anions which are surrounded by water molecules. Substances that do not yield ions when dissolved are called nonelectrolytes.
This is intended as part of an introduction to acids. In summary the correct answer is option a. When table salt NaCl is disolved it is broken up into Na and Cl- -they are pulled apart by water molecules Home.
2 What type of compound is always an electrolyte when dissolved. To dissolve an ionic compound the water molecules must be able to stabilize the ions that result from breaking the ionic bond. The first example that springs to mind is sodium chloride.
1 What compounds form an electrolyte solution in water. Say for example if you dissolve NaCl in water then it decomposes into Na Cl-. Oil contains molecules that are non-polar thus they do not dissolve in water.
-they are broken up into their respective ions. NaCl It will disassociate into its constituent ions Na And Cl- forming an aqueous solution which can conduct electricity. The electronegativity difference between oxygen and hydrogen is high which is why water has a positive pole of H and a negative pole O water is H_2O.
6 Which compound is an electrolyte h2o. What happens is when an ionic compound is put in water the negative ion or the anion attracts the. Cations and anions are released into the solution.
They do this by hydrating the ions. What Happens When an Ionic Compound Dissolves. Na aq Cl aq There are notable exceptions.
When two such solutions are mixed all types of positive ions in the new solution are attracted to all types of negative ions in the solution. NaCl s aq NaCl aq ie. Ionic compounds containing highly polarising ions ones that are small and have a high charge will usually not dissolve in.
Water on the other hand is a polar solvent. A They split up H20 molecules into H and o b Cations and anions are released into the solution c They are transformed into non-metals d They stick to the wall of the beaker. 3 Is anything that dissolves in water an electrolyte.
The water molecules must be able to stabilise the ions that result from splitting the ionic bond to dissolve an ionic compound. These substances constitute an important class of compounds called electrolytes. Does not form any ions in aqueous solutions.
Sometimes a reaction takes place. Characteristics of Ionic and Covalent Compounds. Most ionic compounds dissolve in water because the process is thermodynamically favourable and kinetically accessible.
When an ionic species dissolves in water the ionic compound dissociates into cation and anion and the cation interacts with the negative end of the water molecules and the anion interacts with the positive end of the water molecule. Chemistry questions and answers. If we want to know what happens at a molecular level when a soluble compound is added to water First we have to determine if the soluble compound is an Ionic Compound or Simple Covalent Compound.
This is because water becomes a conductor of electricity. Ionic compounds dissolve in water because the water molecules hydrate the ions.

Dissolving Process Chemistry For Non Majors

Water Also Plays It S Role As The Solvent Of The Body Helping To Regulate The Activity Of Everything It Dissolves And Circula Ionic Compound Solvent Hydration

7 5 Aqueous Solutions And Solubility Compounds Dissolved In Water Chemistry Libretexts

What Happens When Stuff Dissolves Apologia Chemistry Physical Science High School Chemistry
0 Comments